Heat & Energy transfer
Heat & temperature change (4th+ Grade)
Questioning is the foundation of all learning.
The first step in rejecting not knowing is to ask, why?
Sweetland
Science investigation with science inquiry focus on : observation, evidence, variables, measurement, & reasoning from observable evidence to explanation
- Overview
- Big ideas, concepts, facts, & outcomes
- Physical science content concepts & outcomes
- Process - inquiry concepts, facts, & outcomes
- Perspectives
- Pedagogical overview
- Unit activities sequence
- Resources and materials
- Scoring guide suggestions
- Lesson plans
- Activity group 1 - Feeling the heat or cool
- Activity group 2 - Energy transfer - mixing different temperatures and volumes of water
- Activity group 3 - Energy transfer from freezing to boiling and more
- Lab Notes for activities
- Photo journal
Overview
This investigation explores why different objects feel warmer or colder to the touch and investigations for transfer of heat energy in water. Starts with a collection of different objects that learners arrange in order by how warm they feel. Activities continue to help develop the idea that heat energy is transferred by touch from a source to a receiver. Activities provide opportunities for science processes of observation, evidence, variables, measurement, reasoning, evidence, and explanation to be used and discussed. With examples of models for transfer of heat from objects to fingers and model of a window that limits heat transfer with nanotubes.
Activities include: Temperature and sense of touch, energy transfer of mixing water, and energy transfer with freezing and boiling.
Background information:
This plan is designed for students who have the prior knowledge in taking temperature with a thermometer. Suggested Temperature and thermometer activity
Related study topics:
- Cloth, clothes, material, and energy transfer. Science. Sustainable personal Cooling in a Warming World. August 28, 2025.
- Thermometer - fun activity to introduce or practice reading thermometers using thermochromic materials.
- Energy and motion
- Motion - unit of study with spinners, tops, zoomers, twirlers, rolling wheels, rolling cups ,& rolling spheres activities
- Rolling Spheres - inquiry unit that focuses on observation of sphere tracks
- Relative Position & Motion - science unit plan that integrates science content, processss, and inquiry dimensions for observatin, inference, relative position & motion, energy transfer, & force.
- Motion, force and simple machine activities with K'Nex
- Motion roller coasters & ball runs
Pedagogical Overview
Big ideas, concepts, facts, and outcomes
Understanding heat energy is crucial in today's world as it plays a vital role in many aspects of our lives necessary for our survival, enjoyment, and global sustainability.
Heat is a form of energy that we control to be comfortable in our homes and other structures, we use it to creates forces for electricity generation, transportation, and industrial manufacturing and with modernization there is a growing importance of renewable energy sources to supply our increasing demands for cleaner energy sources. To meet out needs it is important to understand how heat is transferred and conserved to improve energy efficiency and reduce greenhouse gas emissions. Moreover, advancements in technology, like thermal management in electronics and climate control systems, rely on a deep knowledge of heat energy. Constructing concepts ofheat and energy transfer is neccessary to combat climate change and fosters the development of innovative, eco-friendly solutions for future energy use.
Heat and clothing
Between 2000 and 2019, heat-related causes claimed over 480,000 lives annually worldwide. Understanding heat is crucial for comfort and protection against extreme weather. Knowing how heat transfers and the four main ways of heating and cooling—radiation, conduction, convection, and evaporation—is vital. Personal heating and cooling technology not only simplifies our lives but also ensures safety in extreme conditions. Construction workers, farmers, firefighters, and athletes face extreme cold and heat, making temperature tech essential to prevent heat stroke, frostbite, and maintain productivity. Smart wearable devices like chest straps and active cooling and heating vests use biosensors and adaptive thermoregulation to keep our body temperature optimal. These sustainable systems offer comfort and health benefits in a rapidly changing climate.
Related concepts and facts
Better decisions are made when information is verified before being considered accurate and used to reason and develop explanations and models to understand the world and make decisions.
Outcome
Use accurate verifiable information related to heat energy, as it relates to the quantity of matter present and its temperature, to explain situations that require the management of heat in climate control in our structures, or for electric generation, transportation, and manufacturing.
Physical Science - Heat Energy, energy transfer, temperature
Big ideas:
What science says about the physical world - enduring understanding, big ideas, generalizations.
- Objects feel warmer or colder to the touch.
- We refer to that sensation as temperature.
- Heat energy is sensed as hot and cold.
- Heat energy is transfered from hot to cold.
- The amount of heat energy in an object determines its temperature.
- Heat energy will transfer from warmer objects to colder objects.
Related concepts and facts
- Heat energy is related to the amount of particle (atom, molecule) movement in a substance.
- Energy is a property of matter that is related to doing something - heat, chemical change, motion, light, magnetism, nuclear change, electrical.
- Heat can be produced in many ways, such as burning, rubbing, or mixing one substance with another.
- Heat is transferred from a source to a receiver.
- Energy is transferred in three general ways (radiation, convection, conduction). Evaporation will also transfer heat energy..
- Conduction is the transfer of energy through matter by particles bumping into each other.
- Convection is the transfer of energy in gases and liquids by the interaction of particles touching. The transfer of energy changes the density of the particles that interact with gravity to create currents.
- Radiation is the transfer of energy through electromagnetic waves. When radiant energy is absorbed it creates heat.
- Heat is transferred in predictable ways, flowing from warmer objects to cooler ones, until both reach the same temperature (equilibrium).
- Heat can be transferred during a chemical, electrical, magnetic, light, mechanical, or nuclear reaction.
- Objects that give off light usually give off heat.
- Mechanical energy is usually related to heat through friction.
- Some materials conduct energy better than others.
- Some materials can transfer heat by contact or at a distance
- Heat is almost always a result of energy transfer.
- Heat can be transferred by touching of particles (collisions of atoms conduction), or through space (by rays radiation) or currents in a fluid (convection).
- Heat energy is the disorderly motion of molecules and in radiation.
- Insulator is a material that has a slower transfer of energy.
- Conductor is a material that easily transfers energy.
- Melting, evaporation, and sublimation of water absorbs energy to causes the water molecules to change their bonding pattern to a higher energy state. On Earth, this energy is transferred from the surrounding environment, which cools the surrounding environment as energy is used to change these phases.
- Condensation, freezing, and deposition of water releases energy to cause the water molecules to change their bonding pattern to a lower energy state. On Earth, this energy is transferred from the surrounding environment, which warms the surrounding environment as energy is used to change these phases.
- Temperature See measurement in math and in science energy concepts.
- See energy in science concepts.
Outcomes
Heat, energy, transfer, & temperature of solids
- Explain heat energy is relates to the quantity of matter present and its temperature.
- Describes hot and cold as a measure of heat energy.
- Describes temperature increases and decreases as directly related to the amount of heat energy.
- Describes heat energy as directly related to the amount of random motion of the particles (atoms,molecules).
- Describes the transfer of heat energy as a change in the random motion of the particles (atoms, molecules) caused by the touching of objects with different amounts of heat energy (conduction).
- Describe energy is transferred in three general ways (radiation, convection, conduction).
- Describe conduction is the transfer of energy through matter by particles bumping into each other.
- Describe convection is the transfer of energy in gases and liquids by the interaction of particles touching. The transfer of energy changes the density of the particles that interact with gravity to create currents.
- Describe radiation is the transfer of energy through electromagnetic waves. When radiant energy is absorbed it creates heat.
- Describes the exchange of heat energy as a product of the temperature differences and mass differences of all the objects involved in the exchange.
- Use observation to compare how different surfaces vary: smooth, rough,bumpy, air pockets... can affect the transfer of heat energy.
- Identify surfaces as a variable that changes from object to object.
- Observe the different surfaces and see if there is a clue on how each might relate to how the energy is being transferred.
- Measure the temperature of different solids.
- Describe the relationship between the kinds of surfaces and the transfer of heat energy.
- Describe and explain that the flow of heat energy between different surfaces is different depending on the surface of the objects.
- Describe how heat transfer affects our daily lives and relates to the world in which we live.
Mixing water
- Measure the temperature of liquids.
- Explain the temperature of the water and the volume of water are both important to understanding the resulting temperature.
- Explain what happens when two equal amounts of water with two different temperatures (like 0 degrees Celsius and 30 degrees Celsius) are mixed together.
- Describe how the temperature of mixing two equal amounts of water with different temperatures will change.
- Describe how the temperature of mixing a small amount of water at one temperature with more water at another temperature will change.
- Use findings to predict how long it will take to cool or heat objects to a certain temperature.
- Use finding to predict how much cold water they need to mix into hotter water to make it a certain temperature.
- Explain when mixing water, the temperature and the amount of the water involved are both important. A single particle (molecules) may have a large amount of heat energy, but if there are not a lot of molecules, there will not be a lot of heat in the entire sample.
Processes, Inquiry, & cross cutting skills to enhance science literacy
Big ideas:
Science is used to explain observations. Observations include our sense of touch, which senses heat energy as hot or cold.
Related concepts and facts
- Inquiry is how science explains the world.
- Observations
- Observations are used to help make explanations.
- Recording observations helps remember specific information.
- People learn by observing interactions with objects.
- Properties of matter can be measured using thermometers.
- Observations are made when someone conducts an experiment.
- Observations are used to help make explanations.
- Properties of objects are determined by the elements from which they are made. Properties can remain constant, change, and be measured. They are used to identify objects, as variables in experiments, operational definitions, and explanations. Properties of matter include: color, texture, size, shape, mass, volume, density, temperature, chemical, energy, states of matter (solid, liquid, gas, plasma) and the ability to interact with other objects. Properties are identified with observation and can be measured with scientific tools and compared to a standard unit (linear, time, temperature, mass, volume, and density)
- Variables
- Variables are conditions that can be changed and that can affect outcomes.
- Variables include, size, shape, temperature, amount, volume, rate, ...
- Measurement is a way to make more accurate or better observations
- Temperature
- Temperature is the measure of heat energy.
- Temperature is measured with a thermometer.
- Thermometer is a tool that measures the temperature (heat energy)
- Water freezes at 0 degree and boils 100 degrees Celsius
- Water freezes at 32 degree and boils 212 degrees Fahrenheit
Outcome
- Observations
- Uses observations to make explanations.
- Variables
- Identify variables and describe how they change. Identify variables and describe how they change (operate to effect other variables - operational definition).
- Identify different variables and describe how they are conditions that can be changed and that can affect outcomes.
- Compare variables. (different surfaces with different shapes and properties, made from different materials that have different properties ...
- Combine variables to see if there are patterns or relationships to use as explanations (variables that affect energy transfer).
- Identify variables and describe how they operate to effect other variables. (operational definition).
- Temperature & thermometer
- States that a thermometer is used to measure temperature.
- Knows the approximate relative temperatures of freezing, room temperature, summer day, and boiling.
- Accurately and safely uses thermometers with different scales to decide an appropriate range or error and measure temperatures from below freezing to boiling.
- Knows how to safely and accurately take appropriate temperature readings and understands that temperature scales are dependent on arbitrary relative references (boiling or freezing) and the degradations one chooses to use between them.
- Model, use accurate verifiable information to make a model that explains the transfer of heat energy to a finger to sense hot and cold.
Perspectives -
Big ideas:
Science can explain our world.
Related concepts and facts
- Physical science relates to all dimensions of Earth science an dlife science.
- Heat exchange is important for understanding Earth science.
- Thermometer's accuracy is dependant on their construction (engineeering).
Outcome
- Describe how energy transfer is important in our world.
Activities Sequence to provide sufficient opportunities for learners to achieve the outcomes
Make sure learners have the prior knowledge identified in the background information.
- Activities - Feeling the heat or cool
- Focus learner's attention by asking the unit focus question, then ask and discuss the sub focus questions and set learning goals.
- Order objects by how cold or warm they feel.
- Discussion
- Take the temperature of the objects
- Discussion
- Temperature and perception
- Look at objects with microscope
- Create a model for possible explanation that explains the observations - both our touching sensations and the observations of the thermometer readings.
- Activities - Energy transfer - mixing different temperatures and volumes of water.
- Solve everyday problems about transfer of heat energy in materials.
- Predict the results of mixing hot and cold water.
- Mix same volumes of water at different temperatures (0C & 30C).
- Mix different volumes of water (250ml & 750ml) at different temperatures (0C & 30C).
- Ice & hot water shower.
- Coffee & milk
- Hot soup.
- Microwave water for tea - how long to save energy?
- Activities - Energy transfer from freezing to boiling and more.
- Melting ice water
- Relate everyday experiences with heat transfer: conduction, convection, radiation in liquids solids, and gases.
- Ice and salt
- Hot and cold bubbles
Focus question
Unit focus question:
What is heat energy and how is it transfered?
Sub focus questions:
- What is temperature? Accept all answers
- What makes objects feel warm and cold?
- What causes your finger to feel a temperature difference?
- Why do some things feel cold and others feel warm?
- How do we determine temperature?
- How can we make better observations?
- How do the observations we make provide more accurate information?
- Could we think of an experiment that we might do to expand our theory of surface shape effecting the transfer of energy?
- How does hot and cold water mix?
Resources and Materials
Interview video of a learner using a temperature probe to measure the temperature of these different objects.
- Activities 1 - Feeling the heat or cool
- Activities 2 - Energy transfer - mixing different temperatures and volumes of water
- Activities 3 - Energy transfer from freezing to boiling and more
Scoring guides suggestions (rubric)
Heat energy transfer (scoring guide)
Top level
- High level: Explains heat is transferred in predictable ways from a source to a receiver. Flowing from warmer objects to cooler ones, until both reach the same temperature. Some materials conduct heat energy better than others, which can effect peoples perceptions of temperature.
- Middle level: Explains heat energy as something different than the object itself. Something that can be transfered from one thing to another.
- Low level: Explains heat with I don't know, or as something with magic like qualities: like heat being a substance continuously generated within the object. Like a blanket, coat, glove, ... generates and gives off heat without consideration of limited supply or conservation of energy.
Lower level
Scoring guide for latent heat transfer
Top level
- High level: Explains latent heat is transferred and used with a change of phase or state of matter to melt, evaporate, sublimate; condense, freeze, and deposit water, without changing temperature. Absorbing energy causes the water molecules to change their bonding pattern to a higher energy state and releasing energy causes the water molecules to change their bonding pattern to a lower energy state.
- Middle level: Explains heat energy won't change the temperature of ice or boiling water, but doesn't explain why.
- Low level: Explains adding or removing heat will change the temperature making objects get hotter or colder.
Lower level
Lesson Plans
Activity - Feeling the heat or cool
Exploration Activity: Order objects from hot to cold by touching them
Materials:
Activity arrange object in order buy temperature
6-8 objects with a range of different surfaces that conduct heat energy from poor to real good: cotton ball, metal, plastic, cloth, chalk, and wood block or pencil; thermometer

Interview video of a learner using a temperature probe to measure the temperature of these different objects.
Materials:
Activity water temperature and perception:
Three cups, hot water, cold water, and medium water.
Focus questions:
- What is the coolest and warmest of the objects?
- Why does a third cup of water feel different temperatures with each finger?
Learning outcomes:
- Arrange a group of objects in order from hot to cold by touch.
Suggested procedures overview:
- Put students in groups, focus their attention, and assess their initial understanding of the focus questions.
- Activity arrange objects in order by sensed temperature.
- Activity feel different temperatures of water and explain.
Suggested procedure - arrange objects in order by temperature:
- Have the collection of objects displayed in the classroom so students can see that they have been there for hours or minutes as appropriate for their coming and going to class.
- Pass one collection of the similar objects to each group and ask them to arrange the objects from the coolest feeling to the warmest feeling and record the results in their lab notes.
- Students should become disequilibrated.
- Discuss with the group, what in their ordering is similar and different.
- Did everyone put the metal cube as the coldest?
- Did everyone put the any other as the warmest?
- Ask students why they feel different.
Suggested procedure - Water temperature and perception:
Materials: Three cups, hot water, cold water, and medium water.
- Place the index finger of your right hand into hot water and the index finger on your left hand into cold water, count to 30, and put both fingers into the medium cup.
- Students should become disequilibrated and will begin to move toward equilibrium during these activities or later during the invention activities.
Invention Activity: Actual temperature of the objects
Materials: Thermometer
Suggested procedure: for looking at objects with microscope.
|metal cube | candle | chalk | wood oak block | plastic cube | fabric | Styrofoam | cotton |
- Take the temperature of each object and write it in your lab notes.
- Have students describe the surface of each object.
- Ask. What is the difference between the surfaces of each?
- Ask. How would your finger interact with each surface differently? The surface area of each object varies from more to less.
- How does the surface area, that touches your finger relate to the temperature?
- What causes your finger to feel a temperature difference?
- Make a model diagram to explain: What causes your finger to feel a temperature difference? OR How the temperatures can actually be identical and feel differently.
- Tell. Your diagram is an Energy transfer model.
- Tell, If you haven't already, Write an explanation.
Model:
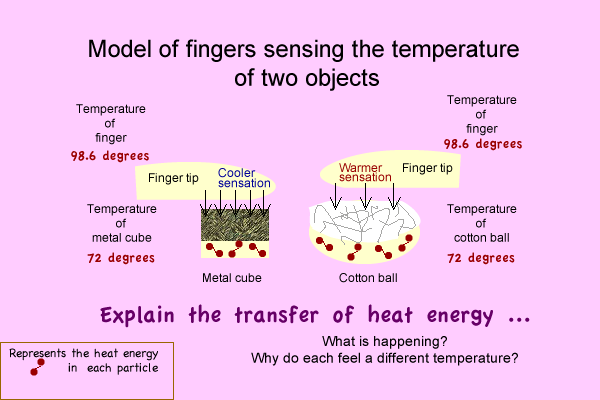
Discover
- How might we use what we learned to make a model of a window that has a coating that doesn't allow the warm air on one side to tranfer to the cold air on the other side? And also the other way around? Accept all answers
- After an appropriate amount of discussion, Suggest to draw a picture of a window.
- Then suggest to label hot and cold on each side.
- Then draw a picture that shows when the cold air touches one side, it bounces or reflects off of it and doesn't pass through.
- Repeat the same for the cold ....
- Ask. If it's a window, how would we show what happens to light. It passes through.
- Share the model of how polysilozane nanotubes added to glass create this.
- Share the
Source:
Invisible heat insulators: A nanotube network with precisely engineered pores could replace insulating components in windows. Longnan Li and Wei Li. Science. December 11, 2025. p 1104.
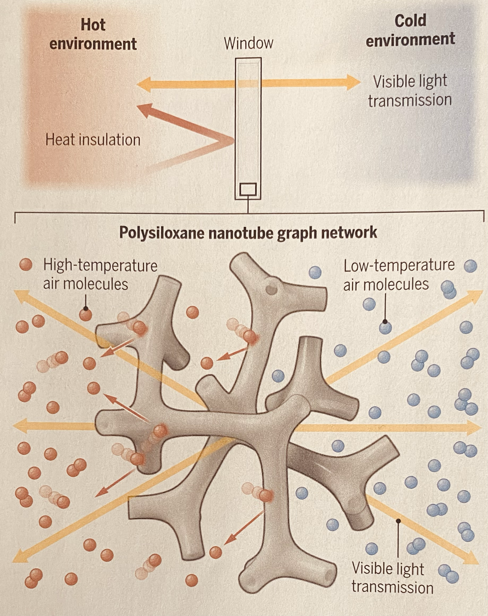
Activity - Energy transfer - mixing different temperatures and volumes of water.
Materials
Per group: Three Styrofoam cups, hot water, cold water with ice, thermometer, paper towels, paper, measuring cup.
Focus questions
- What happens when liquids of different temperatures are mixed?
- How do you decide how to cool or heat things?
- How do you decide how much ice to add to cool things?
- How would you estimate how much cold water needed to add to hotter water to make it a certain temperature?
Learning outcomes
- Exchange of energy by mixing different temperatures of water.
- Solve everyday problems about transfer of heat energy in materials.
- Predict the results of mixing hot and cold water.
- Explain heat energy is relates to the quantity of matter present and its temperature. When mixing water, the temperature and the amount of the water involved are both important. A single particle (molecules) may have a large amount of heat energy, but if there are not a lot of molecules, there will not be a lot of heat in the entire sample.
Suggested procedures overview:
- Mix same volumes of water at different temperatures (0C & 30C).
- Mix different volumes of water (250ml & 750ml) at different temperatures (0C & 30C).
- Ice & hot water shower.
- Coffee & milk
- Hot soup.
- Microwave water for tea - how long to save energy?
Scoring guide for
Top level
- High level: Explains heat is transferred in predictable ways from a source to a receiver. Flowing from warmer objects to cooler ones, until both reach the same temperature. Some materials conduct heat energy better than others, which can effect peoples perceptions of temperature.
- Middle level: Explains heat energy as something different than the object itself. Something that can be transfered from one thing to another.
- Low level: Explains heat with I don't know, or as something with magic like qualities: like heat being a substance continuously generated within the object. Like a blanket, coat, glove, ... generates and gives off heat without consideration of limited supply or conservation of energy.
Lower level
Exploration
- Put students in groups.
- Ask. What happens when liquids of different temperatures are mixed?
- Ask. What happens when two equal amounts of water with two different temperatures (say 0 degrees Celsius and 30 degrees Celsius) are mixed together?
- Let students feel the temperature of water before starting.
- Measure the water (500 ml) into the cups.
- Measure the water temperatures.
- Predict final temperature.
- Mix.
- Record mixed temperatures.
- Share and display all group results (chart or graph) for the class.
Invention
- Discuss the results as a group.
- Compare the results of all of the groups. Average or find the mean.
- Ask. How do you explain what happens when two equal amounts of water with different temperatures are mixed? Produces a temperature midway between the two.
- Ask. What will happen if different amounts of water are mixed?
Discover
- Ask. How can we experiment with different amounts of water to see what happens? Fill two Styrofoam cups with different amounts of water that have different temperatures.
- The small amount (250 ml) about 45 degrees Celsius and the larger amount (750 ml) close to 0 degrees Celsius.
- The small amount (750 ml) about 45 degrees Celsius and the larger amount (250 ml) close to 0 degrees Celsius.
- Other
- Record the temperature of water in each cup.
- Mix.
- Record the mixed water temperature.
- Share, record, and display result for the class.
- Ask. How does the change in temperature of unequal amounts compare to the change in temperature of equal amounts? Temperature change is closer to the temperature of the larger amount (volume) of water.
- Ask. What is more important, the temperature of the water with which you started or the amount of water with which you started? Both are important. Single molecules may have a large amount of heat energy, but if there are not a lot of molecules, there will not be a lot of heat in the entire sample.
Extension activities
Ice and hot water shower
- Tell. Each group to fill five containers with one-fourth cup of crushed ice.
- Tell. Each group to measure out four different amounts of hot water (three-fourths cup, one-half cup, one-fourth cup, and one tablespoon) into other cups.
- Tell. Each group to dump each cup of hot water into one of the cups with the ice.
- After five minutes.
- Tell. Each group to record how much ice is left in each cup. Could weigh (mass) it or measure its volume in tablespoon.
- Tell. Each to record their observations.
- Ask. How the amount of ice left compares to the amount of hot water added to the cup.
- Ask the class to explain, why different amounts of ice were left in each cup when they were all the same temperature? Different amounts (mass or volume) of water was added. If more hot water was added to the ice, it is the same thing as adding more heat energy.
- Optional - Explain heat and temperature are two different properties of materials. Temperature is measured with a thermometer and it indicates the quickness of motion of speed energy each particle (molecule) of water has. Heat energy is a measure of how much energy all the particles in an object have. It shows the total amount of internal energy transferred to or from a specific amount of water (matter).
Generative assessment
Coffee and milk
Mary is having a cup of coffee. She pours herself an almost-full cup of coffee. The temperature of the coffee is about 50 degrees Celsius. She adds a tablespoon of cold milk to the coffee. What temperature is her coffee now? Slightly below. Write and explain your prediction on a piece of paper. A tablespoon of cool milk will not need a lot of heat energy transfered to it until its heat energy (measured by its temperature) is the same as the coffee.
Tell. Describe a procedure to collect evidence that might support your answer.
- Fill two coffee cups (control & experimental) with the same amount of coffee temperature water.
- Measure and record the temperature of both cups of water.
- Pour one tablespoon of milk temperature water into one of the cups.
- Measure and record the temperature of the cups of water.
- Compare the results to the prediction. The temperature should still be close to the starting temperature in both cups.
- Discuss the results.
Hot soup
Jim is thinking about having hot soup for dinner, however he doesn't want it to be too hot to eat and he does not want to wait to eat it. So he is thinking he could start with less water, make the soup, and then add cold water to cool it off to eat without waiting too long. The directions say add one cup of water to start. How much water should he start with and how much will he have to add later? He thinks it should be about 40 degrees Celsius to eat.
What is your prediction and explain why?
How would you design an experiment to test it?
Microwave and heating water
A group in class is wondering if they are wasting energy when they heat water in the microwave. How could they experiment to find out how much time they should microwave a cup, 2 cups, ... of water to boil or reach a temperature ready to drink as tea?
How would you design an experiment to test it?
Activity - Energy transfer from freezing to boiling and more.
Materials
- Per group: Containers (about 1 liter), volume measuring devices, thermometers, ice, cold water, clock or timer.
Focus questions
- How does the temperature of your ice & water (tea, pop, ...) change as the ice melts?
- Dad always said, a slow boil or fast boil will cook the same. What does that mean?
Learning outcomes
- Describe how latent heat energy changes the phase or state of matter without changing the temperature.
Suggested procedures overview:
- Water and ice melt
- Ice water boiling
- Ice and salt
- Conductors & insulators and water temperature change
- Ice cube melting contest
- Ice preservation contest
- Heat energy transfer through metal
- Hot and cold cars in the sun
- Hot and cold drops of water in medium water
- Convection currents in water
- Hot and cold bubbles
- Hot and cold air
Scoring guide for
Top level
- High level: Explains latent heat is transferred and used with a change of phase or state of matter to melt, evaporate, sublimate; condense, freeze, and deposit water, without changing temperature. Absorbing energy causes the water molecules to change their bonding pattern to a higher energy state and releasing energy causes the water molecules to change their bonding pattern to a lower energy state.
- Middle level: Explains heat energy won't change the temperature of ice or boiling water, but doesn't explain why.
- Low level: Explains adding or removing heat will change the temperature making objects get hotter or colder.
Lower level
Exploration activity - Water and ice melt
- Put students in groups.
- Tell groups to put 500 ml of cold water into a container.
- Add about two ice cubes
- Stir slowly
- Record the temperature
- Continue to stir and record the temperature every minute until the ice melts.
- Record results.
- Display results (chart & graph) and share with the class.
- Explain how the results relate to the transfer of heat energy. The transfer of heat from the water to the ice melts the ice, but does not significantly change the temperature of the water as the ice melts. Cool for cooling drinks. (pun intended)
- Ask. How could we increase the temperature of water as the ice melts?
- Maybe if we added more heat as the ice melts?
- How? ... see next
Invention - from ice water to boiling
Materials
For safety, do as a demonstration.
Volume measuring devices, thermometers, ice, cold water, appropriate container for boiling water, hot plate, oven mitts, safe hot surfaces.
Procedure
- Fill an appropriate container with cold water (500 ml).
- Add four ice cubes
- Slowly stir and record the temperature.
- Put the container on the heat source.
- Heat on high enough temperature to bring to a boil.
- Continue to stir and record the temperature every minute until the ice melts.
- Record when the ice melts and continue to stir and record the temperature until the water boils.
- Continue to safely stir and record the temperature while the water is boiling for a minute or two to demonstrate the temperature will not increase.
- Display results (chart & graph).
- Ask. What happens to the heat energy is transferred to the water? The amount of heat energy transferred that does not go into changing the temperature of the water is used to change the state or phase (latent energy changes matter from a solid to a liquid or gas). This energy is important for the water cycle and its effect on climate change. For example snow melting, puddles of water evaporating will use heat energy to change phase or state before warming the underlying surface.
Melting, evaporation, and sublimation of water absorbs energy to causes the water molecules to change their bonding pattern to a higher energy state. On Earth, this energy is transferred from the surrounding environment, which cools the surrounding environment as energy is used to change these phases.
Condensation, freezing, and deposition of water releases energy to cause the water molecules to change their bonding pattern to a lower energy state. On Earth, this energy is transferred from the surrounding environment, which warms the surrounding environment as energy is used to change these phases.
Expansion - ice and salt
Activity - ice and salt
Materials
Gloves, Ice, salt, wood spoon, container, thermometer.
Procedure
- Add layers of ice and salt to a container.
- Handle only with appropriate gloves to protect hands from frost bite.
- Record the temperature.
- Stir.
- Record the temperature every minute for ten minutes. (The sides of the bowl will get very cold and could cause frost bite. Handle the bowls with gloves.)
Activity - conductors and insulators to alter energy transfer
Materials
Three cups made of different materials (Styrofoam, plastic, paper), volume measuring devices, thermometers, hot water, cold water.
Procedure
- Measure the temperature of hot water and cold water
- Measure equal volumes of water into different cups
- Place one of the cups of water into the other cup of water
- Measure the temperature of the hot and cold water every minute for 10 minutes.
- Write a sentence or formula to explain what happened.
Activity - Ice cube melting contest
Materials
For each student - one plastic bag and one ice cube.
Procedure
- Tell each student to put an ice cube in a plastic bag.
- Challenge the students to melt the ice and collect as much water in the plastic bag as they can in 5 minutes. You may want to put some restrictions on materials that they can or can not use depending on the environment.
- Ask. What variables increased and decreased the amount of ice melt?
- Write a summary using the words energy transfer and explain how more or less ice was melted.
Activity - Ice preservation contest
Materials
Ice source and other materials your or the class decides to allow to use to create a device to preserve the ice.
Procedure
- On the designated day give each person or group an ice source.
- Have them put the ice source into their device and set them aside for the day or designated time.
Activity - How does heat energy transfer through metal?
Materials
Safety concerns use oven mitts or pliers and other precautions for light candles.
For a demonstration - four or five birthday cake candles, a metal coat hanger, bigger candle for heat source, safe device to hold metal hanger in a flame, and container of water to cool after experiment.
Procedure
- Melt the bottoms of four or five birthday cake candles enough so they can be attached to a metal rod (coat hanger) about two inches apart.
- Hold one end of the rod with the pliers and put the other end into the candle flame.
- Heat.
- Why do the candles fall off in the order they do?
- Draw, discuss, and explain a model of how energy is transfered.
Activity - Hot and cold cars
Materials
Thermometers, assorted colors of automobiles in the sun for ...
Procedure
- Ask. Are all the cars in the parking lot the same temperature?
- Why or why not?
- They are all in the same air ...
- Discuss where on the car they could take the temperature: outside front hood, outside roof, inside dash, front seat, back seat, in trunk...
- Decide where to take the temperatures of the cars.
- Make a chart with labels for the place on the car, the temperature, the color of the car, and its position.
- May want to discuss how to record its position relative to what? Maybe ask the source of heat energy and suggest relative to the Sun. Consider when to mention: Radiation is the transfer of energy through electromagnetic waves. When radiant energy is absorbed it creates heat.
- Collect data.
- Share, display for class, and discuss.
- Write how energy is transferred to cars sitting in the Sun.
Activity - Hot and cold drops of water
Materials
Per group: Hot and cold water, food coloring, small baby size food jars, eye droppers, smaller medicine cups, paper.
Procedure
- Have each group get:
- One baby food jar filled three-fours with hot water,
- One baby food jar filled three-fourths with cold water,
- One medicine cup filled three-fourths with hot water, and
- One medicine cup filled three-fourths with cold water.
- Add five drops of food coloring to one of the one medicine cup and five drops of another color to the other medicine cup.
- Use the eye dropper to drop drops of the colored hot and cold water into the baby food jars of the hot and cold water.
- Observe how the different temperatures of water interact.
- Draw a diagram for each container of hot and cold water and illustrate what happens to the hot and cold drops in each container.
- Write a summary to explain how the drops interact in the different temperatures of water. Colder drops are more dense and will sink in warmer water. Hotter drops are less dense and will float in cooler water. Convection is the transfer of energy in gases and liquids by the interaction of particles touching. The transfer of energy changes the density of the particles that interact with gravity to create currents.
Activity - Convection currents in water
Materials
Two narrow mouthed glass bottles, cold and hot water, food coloring, plastic or cardboard card.
Procedure
- Fill one glass with hot water and the other with cold.
- Color one glass with food coloring.
- Place the card over the mouth of one container, invert it, and place it onto the cold.
- Observe and record what happens.
- Display results for class to see.
- Discuss how did the results changed if the hot or cold was on the top or bottom.
- Explain the results and write a summary.
Activity - Hot and cold bubbles
Materials
Bubble solution, containers, hot water, cold water, ice, bubble wands for each person or group
Preparation 30-60 minutes before experiment:
- Put the bubble solution in two smaller containers.
- Float one container of bubble solution inside a larger container of hot water (hot water bath)
- Float the other container of bubble solution inside a ;larger container of ice water.
Procedure
- Measure and record the temperature of each bubble solution.
- Tell students to use the hot and cold bubble solution to blow bubbles and observe if there is a difference between hot and cold bubbles.
- Make a chart and describe the kinds of bubbles for each of the solutions.
- What was the temperature or the air where you blew the bubbles?
- Would it make a difference if it was a hot or cold day? Amount of heat energy effects the volume and density of matter.
Activity - Hot and cold air
Materials:
Two containers large enough to partly submerse pop bottles, ice, hot water, pop bottles, balloons.
Procedure
- Put an uncovered bottle into a bucket of hot water for three minutes, do not get water into the bottle but cover it with as much water as possible.
- Put the balloon over the mouth of the bottle.
- Move the bottle from the hot water bucket to the ice water bucket.
- Observe hat happens.
- Repeat the same process, only this time put the bottle into the ice water bucket without the balloon for three minutes, put the balloon on, then place the apparatus into the hot water.
- Observe what happens? Amount of heat energy effects the volume and density of matter, which can be observed, measured, by the expansion or contraction of the air in the bottle balloon system.
Lab Notes
Activity 1 - Feeling the heat or cool
Materials
- Select six different kinds of materials that are at room temperature: not near a heat source, outside window, or in the sunshine.
- Write a general description to identify each in material column of the chart.
- Imagine that you touched each material and predict how warm or cool it would feel if you touched it.
- Now, touch a sample of each and write your observation of how cool or warm it actually felt.
| Material | Prediction | Observation | Comments |
|---|---|---|---|
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 5 | |||
| 6 |
- Which type of material felt the warmest?
- Which type of material felt the coldest?
- Order the materials from warmest to coldest?
Warmest
1.
2.
3.
4.
5.
6.
Coldest
Why do think the materials felt different?
A person made the following statement:
"The different materials do not have different temperatures. Each of the materials is about as warm as all the others even though they felt different."
Why do you think they made this statement?
- What evidence could you collect to come to this conclusion?
Hold a thermometer for about one minute on each of the materials and find all of the temperatures or use a digital thermometer.
- Does the differences match the differences felt?
- What is the air temperature in the room at their original locations?
- What does the data suggest?
Put all the materials in the same place for a period of time. Do you think they will have the same temperature?
- What do you observe?
- What do you conclude from this additional information?
Is it possible that the difference in the temperature felt is caused by some other property, beside temperature?
- Another property of the materials?
- What property do you suspect might be involved?
- Could different materials transfer heat at different rates?
- How do the differences in the temperature relate to the kind of material each is made of?
- For example, does plastic transfer heat less than metal?
- If the plastic would transfer less heat from your hand, then would that
cause it to feel warmer than the metal or colder?
- Would it feel colder the more heat transferred from your hand or warmer?
- Would if feel hotter the more heat transferred from your hand or colder?
- Could the rate of transfer be related to the properties of the different
materials rather than the temperature of the different objects?
- How does the transfer of heat from you hand makes them feel different?
Create a model to explain why the observations - both our touching sensations and the observations of the thermometer readings.
Model diagram:
Extensions
How would you answer the following question posed by a student?
"On a cold day, why do the handlebars of my bike feel colder than the handle grips?"
What kinds of thinking processes did we use to solve the problems.
What processes would scientist say that we used?
| Thinking process | Scientific Process | How did it help or how did we use it? |
|---|---|---|
What feedback from your actions was especially helpful?
Activity 2 - Energy transfer - mixing different temperatures and volumes of water.
Materials:
Three Styrofoam cups, hot water, cold water with ice, thermometer, paper towels, paper, measuring cup.
Focus questions:
- What happens when liquids of different temperatures are mixed?
- How do you decide how to cool or heat things?
- How do you decide how much ice to add to cool things?
- How would you estimate how much cold water needed to add to hotter water to make it a certain temperature?
Procedure:
- Get three cups, thermometer, hot water, cold water.
- Feel the temperature of water before starting.
- Measure ______ (500 ml) of hot water into one cup.
- Measure ______ (500 ml) of cold water into one cup.
- Measure and record the water temperatures.
hot
cold
- Predict final temperature.
- Mix.
- Record mixed temperatures.
Mixed temperature
- Share and display all group results (chart or graph) for the class.
Explain what happens when two equal amounts of water with different temperatures are mixed?
Focus question:
How can we experiment with different amounts of water to see what happens?
- Hints:
- The small amount (250 ml) about 45 degrees Celsius and the larger amount (750 ml) close to 0 degrees Celsius.
- The small amount (750 ml) about 45 degrees Celsius and the larger amount (250 ml) close to 0 degrees Celsius.
- Other
Record the the volume and starting temperatures of hot and cold water.
| Trial 1 | Trial 2 | Trial 3 | Trial 4 | |
|---|---|---|---|---|
| Volume cold | ||||
| Starting temp cold | ||||
| Volume hot | ||||
| Starting temp hot | ||||
| Mixed volume | ||||
| Mixed temperature |

Share, record, and display result for the class.
How does the change in temperature of unequal amounts compare to the change in temperature of equal amounts?
What is more important, the temperature of the water with which you started or the amount of water with which you started?
Ice and hot water shower
Materials:
Five containers, hot water, cold water with ice, thermometer, paper towels, paper, measuring cup.
- Fill five containers with one-fourth cup of crushed ice.
- Measure out four different amounts of hot water (three-fourths cup, one-half cup, one-fourth cup, and one tablespoon) into other cups.
- Pour each amount of hot water into one of the cups with the ice.
- Observe for five minutes.
- Record how much ice is left in each cup.
| Amount of ice left after five minutes | |
|---|---|
| No hot water | |
| Tablespoon hot water | |
| One-fourth cup hot water | |
| One-half cup hot water | |
| Three-fourths cup hot water |
How the amount of ice left compares to the amount of hot water added to the cup.
Why are different amounts of ice left in each cup, when they were all the same temperature?
Coffee and milk
Mary is having a cup of coffee. She pours herself an almost-full cup of coffee. The temperature of the coffee is about 50 degrees Celsius. She adds a tablespoon of cold milk to the coffee. What temperature is her coffee now?
Write and explain your prediction on a piece of paper.
Describe a procedure to collect evidence that might support your answer.
Hot soup
Jim is thinking about having hot soup for dinner, however he doesn't want it to be too hot to eat and he does not want to wait to eat it. So he is thinking he could start with less water, make the soup, and then add cold water to cool it off to eat without waiting too long.
The directions say add one cup of water to start.
How much water should he start with and how much will he have to add later?
He thinks it should be about 40 degrees Celsius to eat.
What is your prediction and explain why?
How would you design an experiment to test it?
Microwave and heating water
A group in class is wondering if they are wasting energy when they heat water in the microwave. How could they experiment to find out how much time they should microwave a cup, 2 cups, ... of water to boil or reach a temperature ready to drink as tea?
How would you design an experiment to test it?
Activity 3 - Energy transfer from freezing to boiling and more
Materials:
- Per group: Containers (about 1 liter), volume measuring devices, thermometers, ice, cold water, clock or timer.
Focus questions:
- How does the temperature of your ice & water (tea, pop, ...) change as the ice melts?
- Dad always said, a slow boil or fast boil will cook the same. What does that mean?
Water and ice melt
- Put 500 ml of cold water into a container.
- Add two ice cubes
- Stir slowly
- Record the temperature
- Continue to stir and record the temperature every minute until the ice melts.
- Display results (chart & graph) and share with the class.
| Temperature | |
|---|---|
| Start temperature | |
| Temperature at 1 minute | |
| Temperature at 2 minutes | |
| Temperature at 3 minutes | |
| Temperature at 4 minutes | |
| Temperature at 5 minutes | |

How do the results relate to the transfer of heat energy?
How could we increase the temperature of water as the ice melts?
Invention - from ice water to boiling
Materials:
For safety, do as a demonstration.
Volume measuring devices, thermometers, ice, cold water, appropriate container for boiling water, hot plate, oven mitts, safe hot surfaces.
Procedure:
- Fill an appropriate container with cold water (500 ml).
- Add four ice cubes
- Slowly stir and record the temperature.
- Put the container on a heat source.
- Heat on high enough temperature to bring to a boil.
- Continue to stir and record the temperature every minute.
- Mark when the ice melts.
- Continue to record the temperature after the ice melts, continue to stir, and record the temperature until the water boils.
- Mark when the water begins to boil.
- Continue to record the temperature for a few more minutes.
- Display results (chart & graph) for the class.
What happens to the heat energy transferred to the water?
| Time | Temperature |
|---|---|
| Start | |
| at 1 minute | |
| at 2 minutes | |
| at 3 minutes | |
| at 4 minutes | |
| at 5 minutes | |
| at 6 minutes | |
| at 7 minutes | |
| at 8 minutes | |
| at 9 minutes | |
| at 10 minutes | |
| at 11 minutes | |
| at 12 minutes |

Expansion activity - ice and salt
Activity - ice and salt
Materials:
Gloves, Ice, salt, wood spoon, container, thermometer.
Procedure:
- Add layers of ice and salt to a container.
- Handle only with appropriate gloves to protect hands from frost bite.
- Record the temperature.
- Slowly stir.
- Record the temperature every minute for ten minutes. (The sides of the bowl will get very cold and could cause frost bite. Handle only with gloves.)
| Time | Temperature |
|---|---|
| Start | |
| at 1 minute | |
| at 2 minutes | |
| at 3 minutes | |
| at 4 minutes | |
| at 5 minutes | |
| at 6 minutes | |
| at 7 minutes | |
| at 8 minutes | |
| at 9 minutes | |
| at 10 minutes | |
| at 11 minutes | |
| at 12 minutes |

Extension activity - conductors and insulators to alter energy transfer
Materials:
Three cups made of different materials (Styrofoam, plastic, paper), volume measuring devices, thermometers, hot water, cold water.
Procedure:
- Measure the temperature of hot water and cold water
- Measure equal volumes of water into different cups
- Place one of the cups of water into the other cup of water
- Measure the temperature of the hot and cold water every minute for 12 minutes.
- Write a sentence or formula to explain what happened.
| Time | Temperature cold | Temperature hot |
|---|---|---|
| Start | ||
| at 1 minute | ||
| at 2 minutes | ||
| at 3 minutes | ||
| at 4 minutes | ||
| at 5 minutes | ||
| at 6 minutes | ||
| at 7 minutes | ||
| at 8 minutes | ||
| at 9 minutes | ||
| at 10 minutes | ||
| at 11 minutes | ||
| at 12 minutes |

Expansion activity - Ice cube melting challenge
Challenge - to melt an ice cube and collect as much water in a plastic bag as you can in 5 minutes.
Materials:
One plastic bag and one ice cube
Procedure:
Describe a strategy you will start with.
How much water did you collect?
What variables increased and decreased the amount of ice melt?
Write a summary using the words energy transfer and explain how more or less ice was melted.
Activity - Ice preservation contest
Materials:
Ice source and device to preserve the ice.
Procedure:
Describe your device and why you believe it will reduce the transfer of energy to melt the ice.
How much ice melted?
Describe the variables in different devices you believed increased and decreased the amount of ice melt?
Write a summary using the words energy transfer and explain how more or less ice was melted.
Activity - How does heat energy transfer through metal?
Materials:
Safety concerns use oven mitts or pliers and other precautions for light candles.
Procedure:
A device with birthday cake candles held on a metal rod with wax will have one end inserted into a heat source.
Predict what will happen.
Describe what happened.
Draw, discuss, and explain a model of how energy was transfered.
Activity - Hot and cold cars
Materials:
Thermometers, assorted colors of automobiles in the sun for ...
Prediction and explanation
When parked in the Sun are the surfaces of a cars in a parking lot the same temperature?
Explain your prediction.
Procedure:
Describe a procedure for taking the temperature of different cars in the parked outside in the sun.
What variables?
All in sunshine for the same amount of time.
Part of car where the temperature is measured: outside front hood, outside roof, inside dash, front seat, back seat, in trunk...
Decide where to take the temperatures of the cars.
Record your procedure.
Collect data and record.
Data chart
| Description of car | Temperature |
|---|---|
Describe how energy is transferred to cars sitting in the Sun.
Activity - Hot and cold drops of water
Materials:
Hot and cold water, food coloring, small baby size food jars, eye droppers, smaller medicine cups, paper.
Procedure:
- Fill two small container three-fourths with hot water.
- Fill two small container three-fourths with cold water.
- Add five drops of food coloring to one of hot water containers and five drops of another color to one of the cold water containers.
- Use an eye dropper to put drops of the colored hot water into the containers of hot and cold water.
- Record the results.
- Use an eye dropper to put drops of the colored cold water into the containers of hot and cold water.
- Record the results.
Diagram what happened to the hot and cold drops in each container.
Hot water with cold and hot drops
Cold water with cold and hot drops.
Write a summary to explain how the drops interact in the different temperatures of water.
Activity - Convection currents in water
Materials:
Two narrow mouthed glass bottles, cold and hot water, food coloring, plastic or cardboard card.
Procedure:
- Fill one container with hot water and the other with cold.
- Color one container with food coloring.
- Place the card over the mouth of the hot container, invert it, and place it onto the cold.
- Observe and record what happens.
- Display results for class to see.
- Discuss how did the results changed if the hot or cold was on the top or bottom.
- Explain the results and write a summary.
Diagram what happened in each container.
Write a summary to explain how the drops interact in the different temperatures of water.
Activity - Hot and cold bubbles
Materials:
Hot and cold bubble solution and bubble wands
Procedure:
- Create hot and cold bubbles and record how they compare.
- Weather cooperating blow bubbles in warm and cold air. (Inside and outside depending on weather)
If there is a difference, describe it and explain what you believe makes a difference.
Activity - Hot and cold air
Materials:
Hot and cold water to partly submerse pop bottles and balloons.
Procedure:
- Put an uncovered bottle into a bucket of hot water for three minutes, do not get water into the bottle but cover it with as much water as possible.
- Put an uncovered bottle into a bucket of cold ice water for three minutes, do not get water into the bottle but cover it with as much water as possible.
- Put a balloon over the mouth of the each bottle (one in hot water & one in cold water).
- Move the bottle from the hot water bucket to the ice water bucket.
- Move the bottle from the ice water bucket to the hot water bucket.
- Observe what happens.
Describe your observations.
Cold to hot bottle
Hot to cold bottle
Use these words to describe what you learned. (heat energy, volume, density, matter, expand, contract, and bottle balloon system.
Photo journal
Thermometer with Celsius and Fehrenheit

Objects to sense (touch) temperature

Model of finger sensing temperatures

Objects to view under magnification
Links to magnified image
|metal cube | candle | chalk | wood oak block | plastic cube | fabric | Styrofoam | cotton |
Model of glass window with optically clear heat insulator








